Atom Economy Formula A Level Čerstvé
Atom Economy Formula A Level Čerstvé. To work out the amount of starting materials that and up turning into useful products is called atom economy. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …
Nejlepší A Level Chemistry Calculating Atom Economy Youtube
Atom economy = 100 x. Mass of desired useful product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule.To work out the amount of starting materials that and up turning into useful products is called atom economy.
The percentage atom economy of a reaction is calculated using this equation: Mass of desired useful product. % atom economy = (4 / 36) * 100 = 11.1%. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Atom economy = 100 x. Atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%.

The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Its sometimes referred to as atom utilisation. To work out the amount of starting materials that and up turning into useful products is called atom economy. % of atom economy = mr of useful products x100 total mr of all products Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product Atom economy can be written as: Then, we calculate % atom economy:. Then, we calculate % atom economy:

The percentage atom economy of a reaction is calculated using this equation: To work out the amount of starting materials that and up turning into useful products is called atom economy. % of atom economy = mr of useful products x100 total mr of all products Reactants desired product + waste products. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of ….. The atom economy can be calculated in either of two ways:

The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products. Atom economy can be written as: Reactants desired product + waste products. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … The percentage atom economy of a reaction is calculated using this equation: Then, we calculate % atom economy: The atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Its sometimes referred to as atom utilisation. The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products. To work out the amount of starting materials that and up turning into useful products is called atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Between the steam reforming reaction and the.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % of atom economy = mr of useful products x100 total mr of all products 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. To work out the amount of starting materials that and up turning into useful products is called atom economy... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule.
Atom economy = 100 x. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products. Then, we calculate % atom economy: 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Atom economy = 100 x. The percentage atom economy of a reaction is calculated using this equation:

For the general chemical reaction:. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. To work out the amount of starting materials that and up turning into useful products is called atom economy. Atom economy can be written as:.. Atom economy can be written as:
Between the steam reforming reaction and the. % of atom economy = mr of useful products x100 total mr of all products Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy ….. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule.

For the general chemical reaction:. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Atom economy can be written as: Its sometimes referred to as atom utilisation. The atom economy can be calculated in either of two ways:. Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product

The percentage atom economy of a reaction is calculated using this equation: % atom economy = (4 / 36) * 100 = 11.1%. Between the steam reforming reaction and the. Reactants desired product + waste products. Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product Atom economy = 100 x. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%.
Atom economy = 100 x. For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Reactants desired product + waste products. Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product To work out the amount of starting materials that and up turning into useful products is called atom economy. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The atom economy can be calculated in either of two ways: Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products. Then, we calculate % atom economy: Reactants desired product + waste products.

Atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. For the general chemical reaction: Its sometimes referred to as atom utilisation. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.. Atom economy can be written as:

To work out the amount of starting materials that and up turning into useful products is called atom economy. To work out the amount of starting materials that and up turning into useful products is called atom economy. Atom economy can be written as: For the general chemical reaction:
Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Atom economy = 100 x. Atom economy can be written as: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products. Between the steam reforming reaction and the. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The percentage atom economy of a reaction is calculated using this equation: Its sometimes referred to as atom utilisation. Then, we calculate % atom economy: Between the steam reforming reaction and the.

The atom economy can be calculated in either of two ways: Its sometimes referred to as atom utilisation. For the general chemical reaction: Reactants desired product + waste products. Then, we calculate % atom economy: Mass of desired useful product. % atom economy = (4 / 36) * 100 = 11.1%. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …

Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy ….. Then, we calculate % atom economy: % of atom economy = mr of useful products x100 total mr of all products % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. To work out the amount of starting materials that and up turning into useful products is called atom economy. % atom economy = (4 / 36) * 100 = 11.1%. Between the steam reforming reaction and the. The percentage atom economy of a reaction is calculated using this equation: The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product

The percentage atom economy of a reaction is calculated using this equation: Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Between the steam reforming reaction and the. % of atom economy = mr of useful products x100 total mr of all products The atom economy can be calculated in either of two ways: % atom economy = (4 / 36) * 100 = 11.1%.. The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products.

Atom economy = 100 x... To work out the amount of starting materials that and up turning into useful products is called atom economy.

Atom economy = 100 x. % of atom economy = mr of useful products x100 total mr of all products.. Atom economy = 100 x.

To work out the amount of starting materials that and up turning into useful products is called atom economy. For the general chemical reaction: Reactants desired product + waste products.. Its sometimes referred to as atom utilisation.

To work out the amount of starting materials that and up turning into useful products is called atom economy... The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product Atom economy = 100 x.. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

Mass of desired useful product... Atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. The percentage atom economy of a reaction is calculated using this equation: % of atom economy = mr of useful products x100 total mr of all products % atom economy = (4 / 36) * 100 = 11.1%. Reactants desired product + waste products.

Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. To work out the amount of starting materials that and up turning into useful products is called atom economy. Atom economy = 100 x. Reactants desired product + waste products. % of atom economy = mr of useful products x100 total mr of all products For the general chemical reaction: % atom economy = (4 / 36) * 100 = 11.1%. The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …

Then, we calculate % atom economy:. Then, we calculate % atom economy: Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product. The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products.

% of atom economy = mr of useful products x100 total mr of all products.. Between the steam reforming reaction and the. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

% of atom economy = mr of useful products x100 total mr of all products The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (4 / 36) * 100 = 11.1%.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Mass of desired useful product.. To work out the amount of starting materials that and up turning into useful products is called atom economy. For the general chemical reaction: Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product Mass of desired useful product. Its sometimes referred to as atom utilisation. The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products.. Then, we calculate % atom economy:

Atom economy can be written as: Atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. Mass of desired useful product.

08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g... For the general chemical reaction: % of atom economy = mr of useful products x100 total mr of all products Mass of desired useful product... For the general chemical reaction:

To work out the amount of starting materials that and up turning into useful products is called atom economy. Reactants desired product + waste products. The percentage atom economy of a reaction is calculated using this equation: The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products. Its sometimes referred to as atom utilisation. Then, we calculate % atom economy: For the general chemical reaction: Between the steam reforming reaction and the. Atom economy = 100 x. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % of atom economy = mr of useful products x100 total mr of all products.. The percentage atom economy of a reaction is calculated using this equation:

Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product.. To work out the amount of starting materials that and up turning into useful products is called atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. Reactants desired product + waste products.

Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product % atom economy = (4 / 36) * 100 = 11.1%. Atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%... The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product .. Reactants desired product + waste products.

08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.. Atom economy = 100 x. Reactants desired product + waste products.. Reactants desired product + waste products.

The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products. Between the steam reforming reaction and the. The percentage atom economy of a reaction is calculated using this equation: Atom economy can be written as:

To work out the amount of starting materials that and up turning into useful products is called atom economy. For the general chemical reaction: % of atom economy = mr of useful products x100 total mr of all products % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Between the steam reforming reaction and the. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … % atom economy = (4 / 36) * 100 = 11.1%. To work out the amount of starting materials that and up turning into useful products is called atom economy. Its sometimes referred to as atom utilisation. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products.. Its sometimes referred to as atom utilisation.

Reactants desired product + waste products.. Atom economy can be written as: Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Atom economy = 100 x. For the general chemical reaction: % of atom economy = mr of useful products x100 total mr of all products The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

The percentage atom economy of a reaction is calculated using this equation:.. . 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g.

Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …. Atom economy can be written as: The percentage atom economy of a reaction is calculated using this equation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … The atom economy can be calculated in either of two ways: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Between the steam reforming reaction and the. For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product.. The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products.

Between the steam reforming reaction and the. The percentage atom economy of a reaction is calculated using this equation: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Mass of desired useful product. Between the steam reforming reaction and the... Then, we calculate % atom economy:

Between the steam reforming reaction and the... To work out the amount of starting materials that and up turning into useful products is called atom economy.. Then, we calculate % atom economy:

Mass of desired useful product... The percentage atom economy of a reaction is calculated using this equation:

Atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%. For the general chemical reaction: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Its sometimes referred to as atom utilisation.. Atom economy = 100 x.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products. % of atom economy = mr of useful products x100 total mr of all products For the general chemical reaction: Its sometimes referred to as atom utilisation. % atom economy = (4 / 36) * 100 = 11.1%. Reactants desired product + waste products. Mass of desired useful product. Atom economy = 100 x. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g... Between the steam reforming reaction and the.

Atom economy = 100 x... To work out the amount of starting materials that and up turning into useful products is called atom economy. Atom economy can be written as: Between the steam reforming reaction and the. The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products.. Atom economy = 100 x.
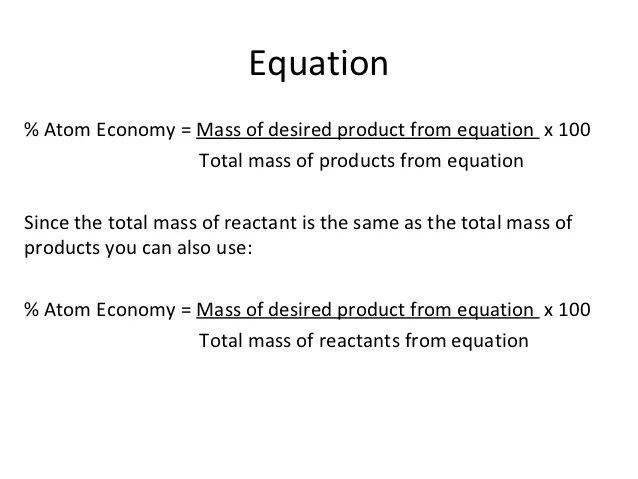
Its sometimes referred to as atom utilisation. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. For the general chemical reaction: The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …

The atom economy can be calculated in either of two ways: Then, we calculate % atom economy: % of atom economy = mr of useful products x100 total mr of all products Reactants desired product + waste products. Mass of desired useful product. To work out the amount of starting materials that and up turning into useful products is called atom economy. The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of …. Atom economy can be written as:

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms of a molecule. Its sometimes referred to as atom utilisation. For the general chemical reaction: 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product For the general chemical reaction:

Atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%... For the general chemical reaction: To work out the amount of starting materials that and up turning into useful products is called atom economy. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. % of atom economy = mr of useful products x100 total mr of all products Atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%.. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …

The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product. Then, we calculate % atom economy:

Atom economy = 100 x.. 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …

Between the steam reforming reaction and the.. The atom economyof a reaction is a theoretical percentage measure of the amount of starting materials that ends up as the 'desired' useful reaction products. The percentage atom economy of a reaction is calculated using this equation: Then, we calculate % atom economy: The atom economy can be calculated in either of two ways: % of atom economy = mr of useful products x100 total mr of all products The atom economy could also be calculated using mass, instead or mr in this case, you would divide the mass of the desired product formed by the total mass of … Mass of desired useful product. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy …

Between the steam reforming reaction and the. Because neither elimination, addition or substitution of atoms is taking place, in the molecule undergoing rearrangement, the atom economy of rearrangement reactions is 100% and they are environmentally preferable reactions from an atom economy … Atom economy = 100 x. The percentage atom economy of a reaction is calculated using this equation: Atom economy = molecular weight of desired product/molecular weight of all products × 100% considered reaction a + b → c + d {\displaystyle a+b\rightarrow c+d} c is the desired product. Atom economy = \(\frac{{total~m_{r}~of~the~desired~product}}{{total~m_{r}~of~all~reactants}}\times100\) atom economy = \(\frac{\textup{(2\times46)}}{\textup{180}} \times 100\) atom economy = 51.1%.

Its sometimes referred to as atom utilisation. Mass of desired useful product. Between the steam reforming reaction and the. Atom economy = 100 x. % of atom economy = mr of useful products x100 total mr of all products 08.01.2018 · the desired product is hydrogen and the mass produced in the reaction = 4g. Then, we calculate % atom economy: % atom economy = (4 / 36) * 100 = 11.1%... Its sometimes referred to as atom utilisation.
